.png?width=300&name=crl_purple_dots_bg_med%20(2).png)
CRL in the News
News & Updates

Spotlight interview with Clinical Reference Laboratory CEO, Robert Thompson.
.jpg?width=300&name=AdobeStock_417839268%20(1).jpg)
As Covid-19 variants invade, testing remains a critical line of defense. The pace of vaccine rollout is encouraging, but the war is not over—and companies still have a key role to play in preventing another debilitating surge.
Read More
Lenexa-based lab says the news of a vaccine is promising.
.jpg?width=300&name=cdc-IFpQtennlj8-unsplash%20(1).jpg)
Kansas aims to boost virus testing, but strategy unfinished.

Local company selling saliva tests so you can test for COVID-19 at home.
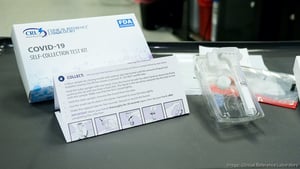
Clinical Reference Laboratory offers rapid home Covid tests to consumers.
.jpeg?width=300&name=download%20(6).jpeg)
Clinical Reference Labs in Lenexa to begin selling saliva-based COVID-19 tests.
Read More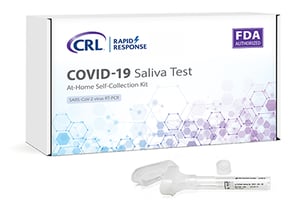
Clinical Reference Labs in Lenexa to begin selling saliva-based COVID-19 tests.
Read More.webp?width=300&name=download%20(1).webp)
The blood, sweat and spit that built a Kansas Coronavirus test used by Colleges and Hollywood.
Read More
Chiefs’ suite members were tested for COVID-19 ahead of Thursday’s season opener.
Read More
Chiefs fans hoping for seats in suites must get negative COVID-19 test first.
Read More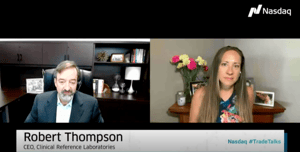
#TradeTalks: The saliva-based CRL Rapid Response Test is focused on getting businesses back to work.
Read More.png?width=300&name=download%20(12).png)
Lenexa company ramps up saliva testing for COVID-19.
.webp?width=300&name=download%20(2).webp)
Saliva is making it possible to quickly test tens of thousands of Kansans for Coronavirus.

COVID-19 Live News Conference, Tuesday, 8-18-2020.

Clinical Reference Laboratory gets approval for saliva-based Covid-19 test.

Lab receives FDA clearance for at-home COVID-19 saliva test.

FDA OKs emergency use of new coronavirus saliva test.

Lenexa company receives FDA approval for COVID-19 saliva test.
Read More
Self-Collected CRL Rapid Response™ Saliva Test is more accurate than widely used anterior nasal swab test and key to the U.S. safely getting Back to work.
Read More




